University Requirements for Disclosing Outside Activities
NSF DisclosuresNIH Disclosures DoD DisclosuresNASA DisclosuresUsing Novelution
Foreign Talent Recruitment Programs
Faculty members and other researchers must disclose financial interests or other activities that might create the perception of or potential for a conflict of interest – both annually and within 15 days of acquiring any new interests, as required by the University Conflict of Interest Policy. They must also disclose or seek prior approval for activities described in the University Conflict of Commitment Policy, including support from foreign governments and foreign academic institutions, consulting relationships, visiting positions, and all investments in start-up companies. Faculty and other researchers must disclose any involvement in any foreign recruitment or “talent” programs. These programs are of particular interest to the federal government, as they are seen as presenting a uniquely high risk of undermining U.S. economic and security interests.
Transparency in Research: Disclosing Activities & Collaborations to Notre Dame and External Sponsors
Federal agencies have established requirements and guidelines for investigators to disclose all forms of research funding, in-kind support of research, and detailed information about significant research collaborations related to federally funded projects. All forms of support must be reported, including in-kind, regardless of where it comes from or where the research is being performed. Federal sponsors are particularly interested in foreign collaborations on federally funded projects. The federal funding agencies are evaluating for:
- Conflict of Interest
- Conflict of Commitment/Available Effort
- Scientific Overlap
Failure to fully disclose foreign/domestic collaborations, affiliations, and resources in funding applications and other documents can have serious consequences and may endanger the University of Notre Dame’s eligibility for future federal funding.
Learn how to ensure all research support and international collaboration are appropriately disclosed to federal sponsors and Notre Dame.
Compliance with the Foreign Corrupt Practices Act, which prohibits payments to foreign government officials to assist in obtaining or retaining business, is also required.
Researchers are required to disclose the following:
- Positions, Appointments, and Affiliations. Any and all (visiting and other) academic, professional or institutional appointments and positions, whether or not remuneration is received, and, whether full-time, part-time, or voluntary
- Financial Support. This includes sponsored awards held at Notre Dame, held at another institution/entity, or held as an individual that supports an investigator’s research efforts. This also includes start-up packages from entities other than Notre Dame, and institutional awards at Notre Dame or other institutions that are separately budgeted and accounted for.
- Non-Financial Resources. This includes non-monetary (in-kind) resources that are uniquely available to key personnel such as office or laboratory space, equipment, supplies, employees, students, scientific materials, and selection to a foreign “talents” or similar-type program. Non-financial resources that are provided by entities other than Notre Dame are of particular interest to the federal funding agencies, and investigators should take care to ensure that all necessary disclosures are made.
- Domestic and foreign consulting relationships
-
Work or travel on behalf of any of the following, whether domestic or foreign.
- academic institutions (other than Notre Dame)
- U.S. and foreign governments
- non-profits/foundations
- industry or trade groups
- private or public companies (including as an investor or any other role)
Disclosures do not need to include travel for the sole purpose of a personal vacation or to attend a conference.
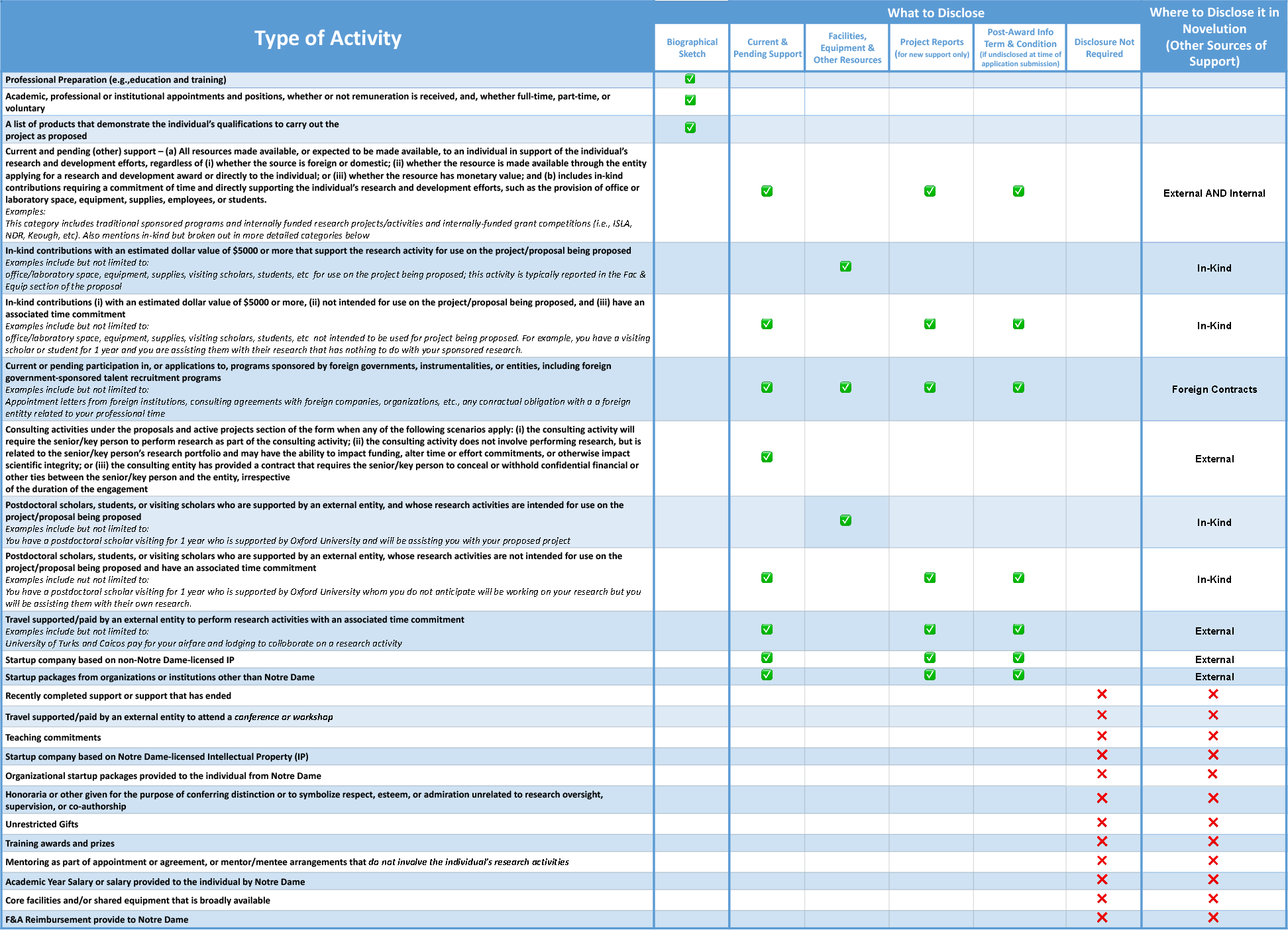
Where to Disclose Research Activities for Federal Sponsors
Three (3) main documents where to disclose this information:
- Biographical Sketch (Biosketch)
- Current & Pending/Other Support (C&P)
- Research Performance Progress Report (RPPR)
Biosketch:
- Professional Preparation - Education and training
- Any and all academic, professional, or institutional appointments and positions - paid or unpaid, full-time, part-time, or voluntary
Current & Pending/Other Support (C&P):
- ALL projects, including projects currently under consideration, from whatever source, and all ongoing projects, irrespective of whether support is provided through Notre Dame, another organization, or directly to the individual and regardless of whether or not they have monetary value (e.g., even if the support received are in-kind contributions such as office/laboratory space, equipment, supplies, or employees)
Research Performance Progress Report (RPPR):
- Anything new or that has changed since proposal submission, JIT, or previous progress report.
Faculty members and other researchers should be thorough and complete in accounting for all forms of research support, including from foreign sources and gifts, in NIH’s Other Support, the NSF's Current and Pending Support, and similar documentation submitted to other sponsors. These disclosures are important, as the University is responsible for disclosing the receipt of foreign gifts and contracts under Section 117 of the Higher Education Act.
Notre Dame Research is pleased to announce the launch of Novelution, a new C&P/Other Support Tool, which is designed to pull information from ND research systems and allow investigators to add additional support that is not found in the ND systems to track their activities required for disclosure to sponsors. Novelution allows investigators to manage the information included in other support documents, store information for future use, and print C&P/Other Support documents in several sponsor formats. NDRAC will review the information provided by researchers in Novelution prior to submitting the C&P/Other Support document to sponsors. Different information is required at different stages by different sponsors (see Federal Sponsor Disclosure Table), so the information captured in Novelution will help streamline the process for everyone involved in the disclosure to sponsor process. To access the Novelution tool click here.
Federal Sponsor Disclosure Table
Need More Information?
For more information on the documents required for proposal submissions, please contact the Pre-Award Team. For more information on progress reports or post-award disclosures, please contact the Grants Program Management team. Both teams may be reached by email researchadmin@nd.edu.