Notre Dame Research FAQs
Notre Dame Research is committed to supporting University faculty in doing what they do best – delivering cutting-edge, globally significant research.
Therefore, Notre Dame Research Administration is available to assist faculty throughout the University in all areas of the research administration lifecycle. These frequently asked questions (FAQs) have been created to help Notre Dame researchers understand the research lifecycle and the services Notre Dame Research offers.
General Research Questions
- How do I find funding in my area of research?
- I will be joining the University as a faculty member. What do I need to do before coming to get my research program up and running?
- I have been approached by someone outside of Notre Dame about to collaborating on a new project. What do I need to do to start work on this research?
- My proposed project requires current space or utilities to be renovated or upgraded. How do I accomplish this as part of my proposal?
- I have a postdoctoral scholar working in my group who would like to submit a proposal. What role do I play in their submission?
- What is cost sharing? Who do I go to for permission to cost share on a proposal?
- What is F&A?
- My research involves human subjects. What do I need to do to ensure I am compliant?
- My research involves animals. What do I need to do to ensure I am compliant?
- The portion of my research that involves either animal or human subjects does not start until the second year of my project. Can I start spending?
- My research involves recombinant DNA or hazardous materials. What do I need to do to ensure I’m compliant?
- I want to ship some supplies to my research site overseas. What do I need to do?
- Does the Fly America Act apply to my international travel?
- Do all proposals require institutional review prior to submission?
- Can I sign an award or agreement letter?
- What do I need to do once I have been approved for funding?
- Is there some way to spend ahead of my project start date?
- How do I know if the purchases I want to make on my grant are allowable?
- What is the difference between capital equipment and supplies?
- I need to hire a specialist for a short period of time to assist me on my research project. How do I do this?
- How can I move expenses on or off an award?
- I need an update on my project’s budget. Where can I get more information?
- I want to extend my grant beyond its end date. What do I need to do?
- My research grant is ending. What do I need to do to ensure that everything is in order to close it out?
- I have a graduate student who received a fellowship. Who should they contact about accessing their funding?
- How do I find funding in my area of research?
The Research Development (RD) Team is available to assist faculty in developing a successful research portfolio. The RD Team provides faculty with expert guidance on connecting to potential resources, collaborators, as well as helping to develop high quality, competitive proposals that lead to success.
Researchers can explore all open funding calls at research.nd.edu/our-services/funding-opportunities/.
Learn more about the Research Development at research.nd.edu/our-services/research-development/.
- I will be joining the University as a faculty member. What do I need to do before coming to get my research program up and running?
Your College or School will send us information regarding your faculty appointment. Once we have this, a member of the NDRA team will reach out to you to discuss the transfer of grants, equipment, postdoctoral scholars, graduate students, and more. In most cases, the coordination of these transfers can happen months before your arrival, helping to facilitate a smooth transition for you and your research program.
However, if you have not been contacted about this please email us or call 574.631.7432.
- I have been approached by someone outside of Notre Dame about to collaborating on a new project. What do I need to do to start work on this research?
Contact your Pre-award Program Manager, who can help you to determine what is needed before the research can begin. Then can assist you with budgeting, sharing relevant information with your colleagues, and any required applications. Please ensure you share any type of research agreement with the Research Contracts team in order for them to have it appropriately reviewed and signed.
- My proposed project requires current space or utilities to be renovated or upgraded. How do I accomplish this as part of my proposal?
You should first discuss the need for additional space, renovation, or utility upgrades for your proposed project with your Department Chair or Dean, as they will need to approve this request prior to proposal submission.
It is important to identify these needs when creating your proposal so that this can be arranged before the space, renovation, or upgrade is needed. Once approved by your Chair or Dean, your Pre-award Program Manager can assist you with documenting the space requirements and approvals needed at the proposal development stage.
- I have a postdoctoral scholar working in my group who would like to submit a proposal. What role do I play in their submission?
Graduate students, postdoctoral scholar, and Visiting Faculty and staff generally do not serve as principal investigators (PI) or project directors (PD), or as co-PIs or co- PDs. To read more, please see the Principal Investigators and Project Directors.
In some cases, an externally funded program may require that one of these individuals be listed as a PI/PD or co-PI/PD or there may be other good cause to waive this restriction. In such cases, your Pre-award Program Manager should be contacted well in advance of the proposal deadline so that the case may be discussed and considered with the appropriate University personnel. A written request may be required from the Chair and Dean of the appropriate department prior to Notre Dame Research approving this status status.
For more information on postdoctoral scholars and their status, please visit postdocs.nd.edu.
- What is cost sharing? Who do I go to for permission to cost share on a proposal?
Cost sharing is the contribution of time or funds to a proposed project. Cost sharing can come from several different sources, but typically originates with the Department, College, or Notre Dame Research. You can read more about cost sharing in the Cost Sharing Guidelines.
If language regarding either mandatory or voluntary cost sharing exists in the sponsor’s proposal guidelines, please notify your Pre-award Program Manager. All requests for cost-share must be approved and recorded in the Cayuse system before the proposal can move forward.
- What is F&A?
F&A stands for Facilities and Administrative costs. These are real costs the University incurs to support research, such as those for utilities, buildings, the Library, and support services, such as purchasing, payroll, and research administration. They are sometimes also referred to as overhead or indirect costs.
F&A rates are approved by the federal government and required on all federal and corporate proposal budgets. If a not-for-profit sponsor, such as a philanthropic foundation, has a limit on the amount of indirect costs that the University can recover, the University will honor the sponsor’s limitation or restrictions on indirect costs, but documentation of this sponsor policy will be required at the time of proposal routing.
You can read more about the Facilities and Administrative Costs or contact your Pre-award Program Manager for further questions.
- My research involves human subjects. What do I need to do to ensure I am compliant?
Notre Dame fosters a research environment that promotes respect for the rights and welfare of individuals recruited for, or participating in, research. Research actions are guided by the principles set forth in the Ethical Principles and Guidelines for the Protection of Human Subjects of Research (“the Belmont Report”) and also conform to all applicable federal, state, and local laws and regulations. To read further, including Notre Dame’s Institutional Human Research Protections Policy, please visit https://research.nd.edu/our-services/compliance/human-research/.
As a result, all faculty and students engaged in human subjects research must submit requests for Institutional Review Board (IRB) review and approval prior to beginning their work. This requirement applies to all such research, regardless of the source of support.
The IRB’s major role is to safeguard the rights and welfare of all human subjects who participate in research. Therefore, in compliance with federal law and institutional policy, all research projects involving human subjects or materials collected from humans (data, samples, etc.) must be reviewed and approved by the IRB before any work can begin. IRB review typically takes up to a month or more, depending on when you submit and when the committee is scheduled to meet.
To submit a request for approval or to track or review information related to animal research, please use the eProtocol system: https://nd.keyusa.net/.
Learn more about human subjects research at Notre Dame, including IRB meeting dates, at https://research.nd.edu/our-services/compliance/human-research/.
- My research involves animals. What do I need to do to ensure I am compliant?
The University of Notre Dame recognizes an inherent obligation that all research utilizing animals is performed with strict adherence to federal guidelines and with the utmost concern for the welfare and well-being of the subjects.
The federal government and University policy require that the care and use of all vertebrate laboratory animals be monitored by the Institutional Animal Care and Use Committee (IACUC). IACUC review and approval is required when animals are used in any research studies and instructional program and must be granted before a research award can be accepted. IACUC review typically takes up to a month or more, depending on when you submit and when the committee is scheduled to meet.
To submit a request for approval or to track or review information related to animal research, please use the eProtocol system: https://nd.keyusa.net/.
Learn more about animal research at Notre Dame, including IACUC meeting dates, at https://research.nd.edu/our-services/compliance/animal-research/.
- The portion of my research that involves either animal or human subjects does not start until the second year of my project. Can I start spending?
If your work involving human or animal subjects will not start until the second year, you can request a memorandum of understanding for a delayed IRB or IACUC approvals with Notre Dame Research Compliance. This will allow you to start spending your grant funds for the non-human or non-animal subject activities prior to receiving this approval.
To initiate this process, please email compliance@nd.edu.
- My research involves recombinant DNA or hazardous materials. What do I need to do to ensure I’m compliant?
Research using these materials, which include, but are not limited to, infectious agents, oncogenic agents, chemical carcinogens, or other agents which may place research personnel, the public, or the environment at risk must be approved by the Institutional Biosafety Committee. Visit https://research.nd.edu/our-services/compliance/biosafety/ or compliance@nd.edu for more details.
- I want to ship some supplies to my research site overseas. What do I need to do?
Contact your Grants Program Manager first. Several offices within the University may need to be involved and your Grants Program Manager will be able to assess the situation and help make this determination, as well as notifying any other necessary departments. Additionally, your Grants Program Manager can offer help in determining if there are additional in-country requirements needed before shipping.
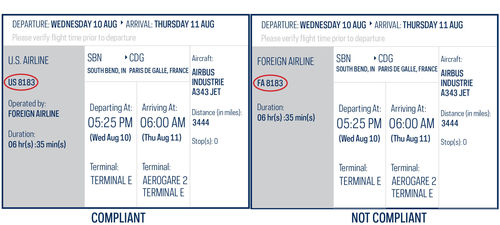
- Does the Fly America Act apply to my international travel?
The Fly America Act is a federal regulation that requires travelers to use U.S. Flag Air Carriers for all foreign travel that is federally funded, regardless of cost or convenience. Some exceptions may apply. Please contact your Grants Program Manager prior to booking a flight on a foreign air carrier.
Code Sharing: Note that U.S. airlines may sell a seat on the plane of a foreign air carrier. In order for a flight to be in compliance with the Fly America Act, the two letter alpha code of a U.S. flag air carrier must be noted as part of the flight number on the airline ticket, boarding pass, or passenger receipt.
- Do all proposals require institutional review prior to submission?
Yes. All proposals must be certified by the principal investigators or project directors and authorized by the relevant Department Chairs, Center Directors, and Deans. This is achieved by electronically through Cayuse, which is the University’s system of record for research administration.
You should discuss sponsor requirements and internal routing procedures with your Pre-award Program Manager before submitting an application to a sponsor.
- Can I sign an award or agreement letter?
No. The University has a small number of delegates who are authorized to sign on behalf of the University and, in most cases, principal investigators do not have the authority to sign these binding agreements.
If you receive a letter of this type, or any other research-related agreement, please send the documents to the Research Contracts Team at rca@nd.edu who will be able to assist you in negotiating the agreement to protect the interests of both the research program and the University and obtaining any appropriate signatures.
- What do I need to do once I have been approved for funding?
If your grant has been properly routed through Cayuse and you have your official notice of award, contact your Grants Program Manager to advise them of the notice. They will then issue you your fund number and you can begin to spend on your project.
Please note that there are a number of issues that could a cause delay at this stage, such as improper routing through Cayuse, pending Compliance issues, or lack of official award documentation. Your Grants Program Manager will be able to advise you on the grant’s status and provide an update on any pending issues.
- Is there some way to spend ahead of my project start date?
This is possible, depending on a number of items. Your Grants Program Manager will be able to assist you in determining this and making the request.
Typically, this is only possible if the formal award document is delayed but there is good documentation that the funds are coming. Some awards do allow spending up to 90 days in advance. Please note that projects with Compliance issues will need to be fully reviewed before any spending can begin.
- How do I know if the purchases I want to make on my grant are allowable?
Your Grants Program Manager should be able to review your award document and tell you what is and is not allowable on your grant.
- What is the difference between capital equipment and supplies?
Supplies are items that cost under $5,000 USD. Equipment is any item that crosses this $5,000 threshold. Further, equipment is defined as having a useful life of more than one year or consists of several smaller items that when combined become a larger piece of constructed equipment that can only function when put together.
- I need to hire a specialist for a short period of time to assist me on my research project. How do I do this?
There are several mechanisms that can be used to pay a subject matter expert or colleague for their contribution. Discuss the situation with your Grants Program Manager so they can help make an informed decision.
You can also learn more by reviewing the Research Consulting Agreement Form here https://research.nd.edu/our-services/resource-library/research-consulting-agreement-form/
- How can I move expenses on or off an award?
It is important that expenses are attributed to the appropriate grant or program. However, there are times when adjustments after a charge has been made are necessary. Such adjustments must be made within 90 days of identifying the error. Please contact your Departmental Support Staff or Grants Program Manager for assistance.
- I need an update on my project’s budget. Where can I get more information?
Your Grants Program Manager has several resources that can assist you with determining financial progress on your project. This team can also create automatic monthly reports for you that can help you to stay-up-date on your project’s financials.
- I want to extend my grant beyond its end date. What do I need to do?
You can request additional time on your grant by completing the No-Cost Extension Form here: https://research.nd.edu/our-services/resource-library/no-cost-extension-form/.
However, not all grants allow an extension. Please contact your Grants Program Manager, who can review your award documentation to determine if this is possible. They can also help you to gather supporting information that can be sent to the sponsor as part of the request.
- My research grant is ending. What do I need to do to ensure that everything is in order to close it out?
Once you receive notice that your award is ready for close out, please contact your Grants Program Manager. Award closeout is a process that requires input from multiple offices and your Grants Program Manager can assist you with this process.
Please note that the technical report is your responsibility.
- I have a graduate student who received a fellowship. Who should they contact about accessing their funding?
Contact the designated Grants Program Manager, to discuss where you are in the process and how to proceed to ensure your graduate student has what they need.
Human Research Questions
- What is research?
- What is a research study?
- What is a research participant?
- What is a protocol?
- What is an Institutional Review Board?
- Can anyone be in a research study?
- What is a Principal Investigator?
- Will I benefit from the research?
- Are there risks to participating in research?
- What is informed consent?
- How do I decide whether or not to participate?
- Is my participation voluntary?
- Do I have rights as a research participant?
- Are there questions that I should ask before agreeing to participate in a research study?
- Are there questions that I my ask myself after the study is over?
- What should parents consider before allowing their child participate in research?
- How can I get more information?
- What is research?
When people are asked about participating in a research study, they may think of clinical trials (drug studies) or other types of medical research, but not all research is medically oriented. Some research studies use questionnaires, interviews, or surveys to gather information on a wide range of subjects, including information about habits, opinions, and beliefs. Other studies observe the way people interact with one another or react to certain situations, providing new insights into human behavior in social or business-related interactions.
- What is a research study?
A research study is a pre-designed and structured way of analyzing behavioral or bio-medical questions, collecting data, and analyzing the data. It can be something as simple as asking questions, giving a survey, or looking at a particular behavior. On the other hand, it can be more complicated and may look at a specific disease or condition.
- What is a research participant?
A research participant (also referred to as a research subject) is an individual who decides to participate in a research study. Participation is completely voluntary. You can decide not to participate or, if you start participating in a study, you can stop participating any time you want.
- What is a protocol?
All research studies follow a protocol or plan of how the research will be done. A protocol tells the researcher what can and cannot be done when he/she is conducting the study. All of this is done to make the study scientifically valid and to help protect the research participant. An Institutional Review Board must review research studies and protocols.
- What is an Institutional Review Board?
The Institutional Review Board (IRB) is made up of a group of people including scientists, non-scientists, ethicists, and some people from the local community. The IRB looks closely at every protocol or research study before it is allowed to begin. Because research studies involve risk, the IRB looks at the study to make sure the risks are justified and minimized as much as possible.
- Can anyone be in a research study?
- The protocol or plan for every research study defines, for scientific and safety reasons, who can and cannot be a participant. In order to protect research subjects, only people who meet the criteria defined in the research protocol are allowed to be in the study.
- What is a Principal Investigator?
The Principal Investigator (PI) is the person who is responsible for the conduct of the research study. He/she is responsible for making sure everything is done properly and according to the research protocol. In addition to the PI, there may be other researchers or staff who help with the study.
- Will I benefit from the research?
In one way or another, all of us have indirectly benefited from the knowledge gained from research. The vaccines that help prevent diseases and the medications that we take are just a few examples of scientific discoveries that resulted from research. Many of the psychological and social support services that we routinely provide to victims of trauma and disaster also were developed in response to research findings. Whether there is a possibility that you will directly benefit from the research that you participate in depends on the type and intent of the research. You may or may not directly benefit from participation but there almost always is a benefit to society.
- Are there risks to participating in research?
- It is important to note that research is experimental and that means it involves risk, sometimes more than you would experience in a normal day. Federal regulations require researchers to inform participants about the risks involved – and to do everything possible to minimize those risks. However, risk can never be completely avoided.
- What is informed consent?
When you are deciding whether or not to volunteer in a research study, the facts of the study may be given to you in a consent form and/or described to you verbally by the researcher. This process is intended to help you understand exactly what you will be required to do or what will happen to you in the research study. It is intended to help you make up your mind about whether or not to participate in the study.
The informed consent process and/or document will outline all the rights you have as a volunteer in a research study. You will also be told about the known or expected risks and any potential benefits that may exist. After reading the consent form and having the research answer your questions, you can make your final decision about participating in the proposed study. It is important that you be given enough time to ask as many questions as you want to about your voluntary participation in the study.
You should not sign the form agreeing to the research until all of your questions have been answered to your satisfaction. Signing this form does not waive any of your legal rights or alter your ability to stop participating at a later time. You should be given a copy of the form to take with you in case you have questions later.
- How do I decide whether or not to participate?
The decision to participate in a research study is a personal one. It may be helpful to discuss your options with researchers and your family but ultimately the choice is yours. There are a number of reasons why people choose to participate in research, but no matter what the reason might be, it is important that your decision not be made lightly.
- Is my participation voluntary?
Yes, participation in a research study is completely voluntary. You are free to decline to participate for any reason or, if you are participating, you may stop at any time. If the research involves a survey or interview, you may refuse to answer any individual questions. Even after you sign the consent form, you can stop. Should you decide to decline or stop participating, this decision will in no way influence any services or benefits to which you are otherwise entitled.
- Do I have rights as a research participant?
Yes, you have the following rights as a research participant:
- To be treated with respect, including respect for your decision whether or not you wish to enroll in, continue in, or stop being in a study.
- To choose to stop being in a study at any time.
- To be given time to read the consent form and have the research study explained to you.
- To be given time to ask questions and to be told whom you can contact if you have any more questions.
- To be given a copy of the consent form after you have signed it.
- Are there questions that I should ask before agreeing to participate in a research study?
Yes, you may want to ask some or all of the following questions when deciding to participate:
- Why is the research being done?
- Does anyone review the research before it begins?
- When will this research take place?
- Where will the research take place?
- What will be done to me or what will I have to do as part of the research?
- How long will my participation last?
- How will I benefit from the research?
- If not me, who will benefit from the research?
- Could the research hurt me?
- What will the researcher do with my personal information or other data obtained through my participation in the study?
- How long will the study last?
- Will the results of the research be disseminated and/or published?
- Can I contact the researcher after the research begins if I have questions?
- Is there anyone, other than the researcher, that I can contact if I have questions or concerns?
- Are there questions that I my ask myself after the study is over?
Yes, you might want to consider:
- Would I be willing to participate in other research studies if asked?
- Would I recommend research participation to others?
- What should parents consider before allowing their child participate in research?
Many types of research studies ask to include children as participants. Some examples are studies about learning styles, early language development, and social development. Children are considered a vulnerable population because their psychological, physical, social, and cognitive capacities are not fully developed and, because they are vulnerable, special ethical and regulatory considerations are required to protect their rights.
The risk levels considered during review of research studies may be minimal risk (classroom observation, curriculum evaluation, or standardized testing evaluation, for example) or greater than minimal risk (i.e. sensitive data collection or therapeutic interventions). Benefits to the children are also considered during the review. For minimal risk studies, the direct benefits do not have to be that high. However, for greater than minimal risk studies, the research results should present a reasonable opportunity to further our understanding of our ability to prevent or alleviate a serious problem affecting the health or well-being of children.
You may be asked for permission to allow your child to participate in a research study. If your child is able to comprehend the procedures and risks of participation, s/he will have to assent to participation. It is the researcher’s responsibility to provide you and your child with information about the research study. It is your responsibility to weight the risks and benefits of the research study and ask questions about the study and your child’s involvement. The parental permission form that you will be asked to sign should list the things that will be done in the research and the risks and potential benefits of the study, but you can ask additional questions.
Questions that must be answered before you provide permission for your child to participate are:
- What will your child be asked to do?
- How much time will it take?
- What are the risks of participation?
- What are the potential benefits of participation?
- How will my child’s identity and the data collected be protected?
- Does my child want to participate?
- How can I get more information?
There are general research brochures that you can get from Federal Regulatory Agencies by visiting the following links:
Office of Human Research Protections (English version): http://www.hhs.gov/ohrp/sites/default/files/ohrp/education/brochures/3panelfinal.pdf
Office of Human Research Protections (Spanish version): http://www.hhs.gov/ohrp/sites/default/files/ohrp/education/brochures/ohrp3panelspanish.pdf
Food and Drug Administration (English): http://www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM390869.pdf